IVDR-compliant device and analysis software
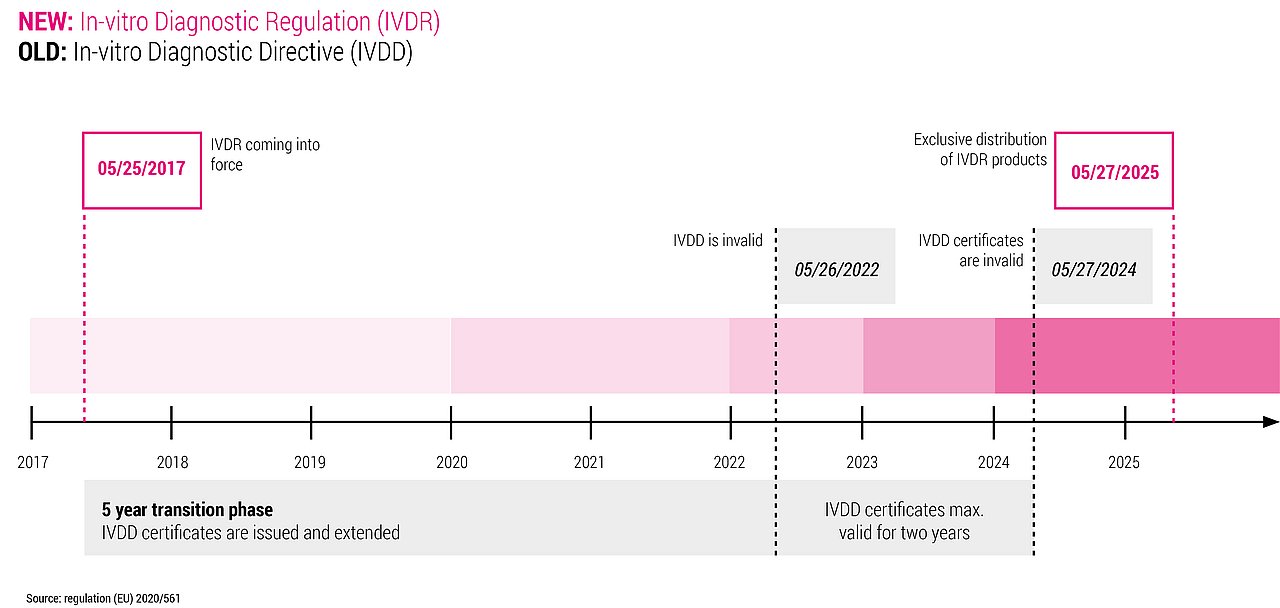
From May 26, 2022, the regulation (EU) 2017/746 of the European Parliament and of the Council of in-vitro diagnostic medical devices will apply. When placing in-vitro diagnosics on the market, medical device and software providers shall comply with this new regulation. We are experts in developing IVDR-compliant device and analysis software for the all standards, such as IEC 62304 – software life cycle, ISO 14971 – risk management and IEC 62366 – usability. This know-how ensures your fast, inexpensive and rulecompliant market access.
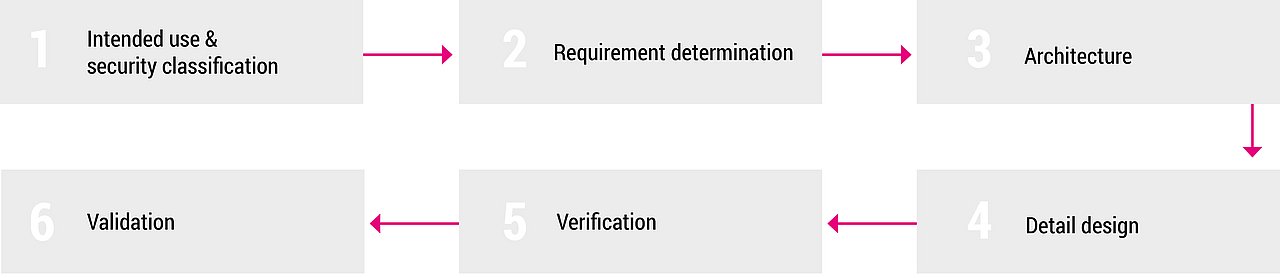
Step-by-step to your IVDR-compliant solution
Our expertise is our well-coordinated team of professionals. From software developers, biologists, mathematicians, physicists to forensic scientists and supply chain management experts. Regardless of which dedicated solution is required, we create targeted results with well-planned processes through all manufacturing steps, from consulting to validation, for new developments and customization of existing software:
We overcome all obstacles
- IEC 62304 medical device software - software life cycle processes
- IEC 62366 usability of medical devices
- ISO 14971 risk management for medical devices
For state-of-the-art IT security.
We are always looking for a challenge. Get in touch with us, so we can help you to meet your legal requirements for product safety.